Advantages of automated medical device reprocessing over manual methods
Automation controls key disinfection variables
When it comes to disinfecting ultrasound probes, automation controls key variables that can impact efficacy whereas manual methods are primarily user-dependent. As such, an ultrasound probe disinfection solution that provides validated automated disinfection constitutes best practice.3
|
Key Variable |
trophon Technology |
Manual HLD Soaks with OPA/GTA |
Manual HLD Wipes |
|
Disinfectant contact time |
Automated & Controlled |
Manual with user-dependant variables4-6 |
Manual with user-dependant variables7 |
|
Disinfectant dose |
Automated & Controlled |
NA |
Manual with user-dependant variables7 |
|
Mechanism of disinfectant coverage |
Automated H2O2 mist |
Chemical immersion4-6 |
Manual wiping7 |
|
Built-in digital traceability of process parameters |
Yes - Automated |
No – Manual5-6 |
No – Manual7 |
Automation improves adherence outcomes
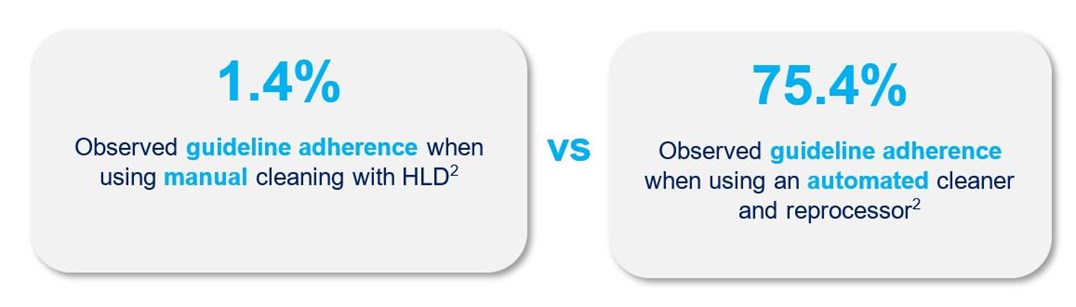
A difference in guideline adherence was found when comparing two methods of endoscope reprocessing: manual cleaning followed by automated high-level disinfection, and an automated technology that standardizes both cleaning and HLD.2

| Efficacy | Automation with trophon technology controls the disinfectant contact time, dose, and coverage to reproducibly achieve high-level disinfection across every trophon HLD cycle. With AcuTrace® technology, the trophon2 device captures disinfection traceability requirements for you, aiding audit readiness. |
| Workflow | Automation with trophon technology streamlines your workflow by limiting hands-on time to reprocess ultrasound probes, giving you time to care for patients and complete other important tasks like close out an exam, review images with a radiologist or prepare the room for the next patient. trophon automated technology enables you to easily standardize your ultrasound probe reprocessing training and process across your facility. |
| Safety | trophon device features an enclosed system protecting users and patients by limiting exposure to harmful chemicals. It can be placed in and used at point-of-care like in an examination room without the need for special ventilation or eyewash station requirements or personal protection equipment (PPE), beyond gloves. This also removes the need to transport probes for reprocessing, speeding up throughput and reducing the need for a large probe inventory. |
Achieving high-level disinfection via manual methods is dependent on multiple user variables that can impact efficiency.1,2 Manual methods also potentially expose users to harmful chemicals.4-7,9,10
Three quarters of healthcare providers reported feeling pressure to work quickly when reprocessing medical devices such as endoscopes.2 This pressure can result in skipped or rushed steps, resulting in a less effective process.2 Additionally, manual HLD methods are open systems that can expose staff to potentially harmful vapors.9,10 Sonographers view automated technologies for ultrasound probe HLD as being more efficient, and safer than manual processes.1
Find out how easy it is to integrate trophon2 into your practice
- Johnson S et al. Evaluation of a hydrogen peroxide-based system for high-level disinfection of vaginal ultrasound probes. J Ultrasound Med 2013; 32:1799–1804.
- Ofstead et al. Endoscope Reprocessing Methods. Society of Gastroenterology Nurses and Associates 2010; 33:304-311.
- Bradley CR et al. Guidance for the decontamination of intracavity medical devices: the report of a working group of the Healthcare Infection Society. Journal of Hospital Infection 2019; 101(1):1-10.
- Civco GUS IFU 2021
- ASP Cidex OPA IFU, Jan 5, 2021
- Regimen GTA IFU, Accessed Dec 2023
- Tristel ULT IFU Aug 8, 2023
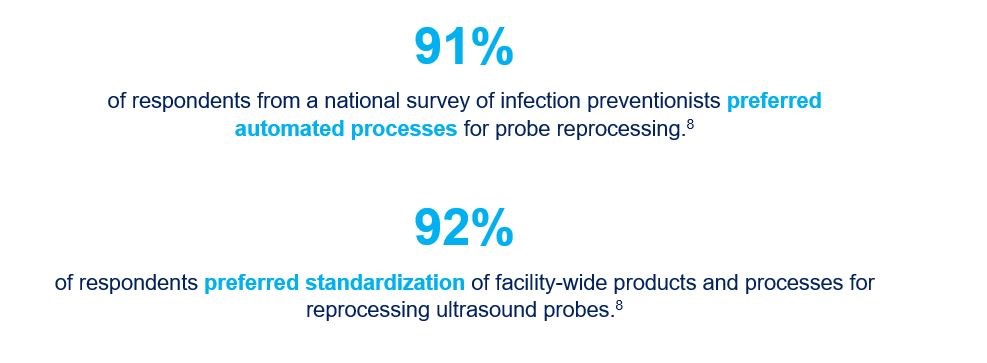
- Carrico RM et al. Ultrasound probe use and reprocessing: Results from a national survey among U.S. infection preventionists. Am J Infect Control 2018; 46(8):913-920.
- OSHA 29 CFR 1910.151(c)
- OSHA PELs, 29 CFR 1910.1000(a)(2)


