Every day, 106,000 patients are protected from the risk of cross contamination because the ultrasound probe has been high-level disinfected with the trophon® device.
Since the trophon device’s launch in North America in 2011, there have been over 28,000 trophon devices installed throughout healthcare facilities across North America. This equates to patients being protected from the risk of cross-contamination in more than 100 million procedures. But this is only the start. Millions of procedures remain unprotected by trophon technology. trophon technology has redefined the global standard of care in ultrasound probe reprocessing, providing fail-safe ultrasound probe high level disinfection (HLD).
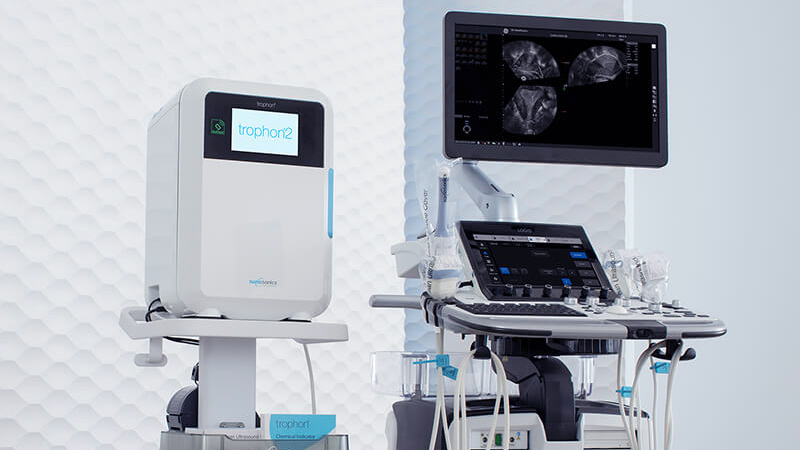
With the introduction of the trophon®2 device, the world's best just got better. The trophon2 device has an enhanced user experience for greater workflow efficiencies and traceability across the workflow for demonstrated compliance, to deliver consistent patient protection with every automated HLD cycle.

The trophon device is the only automated HLD technology for transvaginal, transrectal and surface probes to meet mandatory microbial efficacy test requirements for both CE mark and FDA registration.
In addition to mandatory testing, the trophon device is demonstrated to eliminate an extended range of clinically relevant pathogens including multi-drug resistant bacteria, blood borne viruses and sexually transmitted pathogens.
AcuTrace® RFID technology simplifies the creation of accurate digital records across the reprocessing workflow to support audit readiness.
With the trophon2 device and AcuTrace, users can captures the full reprocessing workflow electronically, removing the need for manual record keeping which is time consuming and error prone.
The trophon2 device is a fully enclosed, automated HLD system that saves you time with a simple, fast and integrated workflow solution. The trophon2 device makes point of care reprocessing possible, offering a solution that has been designed specifically for patient examination rooms.
The trophon2 dedvice is simple to operate and with minimal training, new staff members can confidently deliver consistent HLD of your ultrasound probes. With new and improved features, the trophon2 device can be configured so it is always ready when you are.
Nanosonics, in collaboration with ultrasound probe manufacturers, has developed and implemented an industry leading probe compatibility program. Only probes approved by the probe manufacturers are listed as compatible with the trophon device.
Today, over 1,300 probes from all major and many specialty probe manufactures have been approved as compatible with the trophon device.
In addition to HLD, the trophon device portfolio offers a number of consumable and accessory product solutions to allow users to prepare, disinfect, track and trace, and store ultrasound probes therefore offering a complete reprocessing solution.
The trophon® family includes trophon® EPR and trophon®2 which share the same core technology of 'sonically activated' hydrogen peroxide.
Find out how easy it is to integrate trophon2 into your practice
*The trophon® family includes trophon® EPR and trophon®2 which share the same core technology of sonically activated hydrogen peroxide.

